- Lewis Dot Structure 5a
- Lewis Dot Structure For Nh3
To draw Lewis dot structures, start by writing the atomic symbols for the 2 atoms side-by-side. Then, determine whether the atoms are held together by a single, double, or triple bond. Next, draw lines between the atoms to represent that bond. For example, use 1 line to show a single bond, or draw 2 lines if they have a double bond.
See full list on vedantu.com. To generate the Lewis dot structure, you have to follow the given steps: Find the total count of valence electrons to molecules. In this step, add the total count of valence electrons from all the atoms in a bit. Find the required count of electrons needed to make the atoms complete. After then, define the number of bonds in the given molecule. WRITING LEWIS DOT STRUCTURES Lewis structure or formula shows electron‐dot symbols for the atoms, the bonding pairs as lines, and the lone pairs that fill each atom's outer level (valence shell) as pairs of dots. Determine the total number of valence electrons. Add up the valence electrons of the atoms.
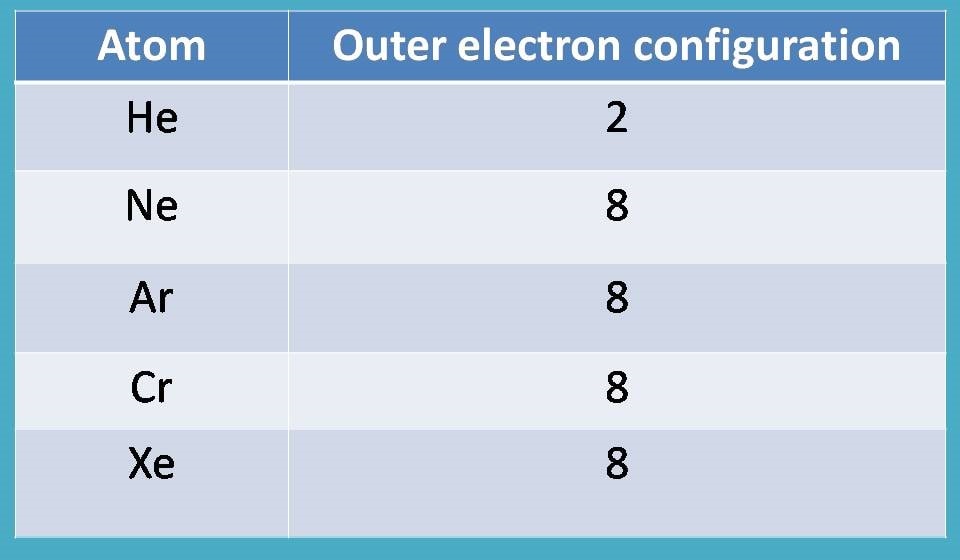
A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. As electrons are added, they fill electron shells in an order determined by which configuration will give the lowest possible energy. The first shell (n=1) can have only 2 electrons, so that shell is filled in helium, the first noble gas. In the periodic table, the elements are placed in 'periods' and arranged left to right in the order of filling of electrons in the outer shell. So hydrogen and helium complete the first period.
The number of electrons in a given shell can be predicted from the quantum numbers associated with that shell along with the Pauli exclusion principle. The second shell, associated with principal quantum number n=2, can have a maximum of 8 electrons and corresponds to the second period of the periodic table. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them.
| Lewis Dot Diagrams | Visualization of electron orbitals |
 Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice.
Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice. Lewis Dot Structure 5a
Lewis Structures are important to learn because they help us predict:

- the shape of a molecule.
- how the molecule might react with other molecules.
- the physical properties of the molecule (like boiling point, surface tension, etc.).
That helps us understand and predict interactions with things like medicine and our body, materials used to make buildings and airplanes, and all sorts of other substances. Lewis structures don't tell us everything, but along with molecule geometry and polarity they are hugely informative.
Search 100+ Lewis Structures on our site.
(Opens new window.) |
Click the Chemical Formula to see the Lewis Structure | Acetone | (C3H6O) | | AsCl3 | (Arsenic Trichloride) | | AsF3 | (Arsenic Trifluoride) | | AsF5 | (Arsenic Pentafluoride) | | AsF6- | (AsF6-) | | AsH3 | (Arsenic Trihydride) | | AsO33- | (Arsenite Ion) | | BBr3 | (Boron Tribromide) | | BCl3 | (Boron Trichloride) | | BF3 | (Boron Trichloride) | | BF4- | (Tetrafluoroborate Ion) | | BH3 | (Boron Hydride) | | BH4- | (BH4-) | | B(OH)3 | (B(OH)3) | | BeCl2 | (Beryllium Chloride) | | BeF2 | (Beryllium Fluoride) | | BeH2 | (Beryllium Hydride) | | Br2 | (Bromine Gas or Elemental Bromine) | | Br3- | (Tribromide Ion) | | BrF | (Bromine Monofluoride) | | BrF2 | (Bromine Difluoride) | | BrCl3 | (Bromine Trichloride) | | BrF3 | (Bromine Trifluoride) | | BrF5 | (Bromine Pentafluoride) | | BrO- | (Hypobromite Ion) | | BrO2- | (Bromite Ion) | | BrO3- | (Bromate Ion) | | C22- | (Dicarbide Ion) | | CBr4 | (Carbon Tetabromide) | | CCl4 | (Carbon Tetachloride) | | ClF | (Chlorine Monofluoride) | | CF2Cl2 | (Dichlorodifluoromethane) | | CH2Cl2 | (CH2Cl2) | | CH3- | (CH3-) | | CH3Br | (CH3Br) | | CH3Cl | (Chloromethane or Methyl Chloride) | | CH3CN | (Acetonitril or Methyl Cyanide) | | CH3COO- | CH3COO- | | CH3COOH | (Acetic Acid) | | CH3F | (CH3F) | | CH3NH2 | (Methylamine) | | CH3NO2 | (CH3NO2) | | CH3OCH3 | (Dimethyl Ether or Methoxymethane) | | CH3OH | (Methanol or Methyl Alcohol) | | CH4 | (Methane) | | C2F4 | (C2F4) | | C2H2 | (Ethyne or Acetylene) | | C2H2Br2 | (C2H2Br2) | | C2H2Cl2 | (C2H2Cl2) | | C2H4 | (Ethene) | | C2H6 | (Ethane) | | C2H6O | C2H6O | | C3H6 | (C3H6) | | C3H8 | (Propane) | | C4H10 | (Butane) | | C6H6 | (Isomers - including Benzene) | | C6H12 | (C6H12) | | CHCl3 | (Chloromethane) | | CH2F2 | (Difluoromethane) | | CH2O | (Methanal or Formaldehyde) | | CH4O | (CH4O) | | Cl2 | (Chlorine Gas or Elemental Chlorine) | | Cl2CO | (Cl2CO) | | Cl2O | (Dichlorine Monoxide) | | Cl3PO | (Phosphoryl Trichloride) | | ClF3 | (Chlorine Trifluoride) | | ClF5 | (Chlorine Tetrafluoride) | | ClO- | (Hypochlorite Ion) | | ClO2 | (Chlorine Dioxide) | | ClO2- | (Chlorite Ion) | | ClO3- | (Chlorate Ion) | | ClO4- | (Perchlorate Ion) | | CO | (Carbon monoxide) | | CO2 | (Carbon Dioxide) | | CO32- | (Carbonate Ion) | | COCl2 | (COCl2) | | COF2 | (COF2) | | COH2 | (COH2) | | CN- | (Cyanide Anion) | | CS2 | (Carbon Disulfide) | | F2 | (Fluorine Gas, Difluorine) | | H2 | (Hydrogen Gas or Elemental Hydrogen) | | H2CO | (Formaldehyde or Methanal) | | H2CO3 | (Carbonic Acid) | | H2O | (Water or Dihydrogen monoxide) | | H3O+ | (Hydronium Ion) | | H2O2 | (Hydrogen Peroxide or Dihydrogen Dioxide) | | HBr | (Hydrogen Bromide or Hydrobromic Acid) | | HF | (Hydrogen Fluoride or Hydrofluoric Acid) | | HCCH | (Ethyne) | | HCl | (Hydrogen Chloride or Hydrochloric Acid) | | HCO2- | (Formate Ion) | | HCO3- | (Hydrogen Carbonate Ion or Bicarbonate Ion) | | HCOOH | (Methanoic Acid or Formic Acid) | | HI | (Hydrogen Iodide or Hydroiodic Acid) | | HClO3 | (Chloric Acid) | | HCN | (Hydrogen Cyanide) | | HNO2 | (Nitrous Acid) | | HNO3 | (Nitric Acid) | | H2S | (Dihydrogen Sulfide) | | HOCl | (Hypochlorous Acid) | | H2Se | (Dihydrogen Selenide) | | HSO3- | (Bisulfite Ion) | | HSO4- | (Bisulfate Ion) | | H2SO3 | (Sulfurous Acid) | | H2SO4 | (Sulfuric Acid) | | H3PO4 | (Phosphoric Acid) | | I2 | (Iodine Gas or Elemental Iodine) | | I3- | (I3-) | | IBr2- | (IBr2-) | | ICl | (Iodine Chloride) | | ICl2- | (ICl2-) | | ICl3 | (ICl3) | | ICl4- | (ICl4-) | | ICl5 | (Iodine Pentachloride) | | IF2- | (IF2-) | | IF3 | (Iodine Trifluoride) | | IF4- | (IF4-) | | IF5 | (Iodine Pentafluoride) | | IO3- | (Iodate Ion) | | IO4- | (Perioiodate Ion) | | N2 | (Nitrogen Gas, also called Elemental Nitrogen) | | N3- | (Azide Ion) | | N2F2 | (Dinitrogen Difluoride) | | N2H2 | (Dinitrogen Dihydride) | | N2H4 | (Dinitrogen Tetrahydride or Hydrazine or Diamine) | | N2O3 | (Dinitrogen Trioxide) | | N2O4 | (Dinitrogen Tetroxide) | | N2O5 | (Dinitrogen Pentoxide) | | NCl3 | (Nitrogen Trichloride) | | NF3 | (Nitrogen Trifluoride) | | NH2- | (NH2-) | | NH2Cl | (Chloroamine) | | NH2OH | (Hydroxylamine) | | NH3 | (Ammonium or Nitrogen Trihydride) | | NH4+ | (Ammonium Ion) | | NI3 | (Nitrogen Triiodide) | | NO+ | (Nitrosonium Ion) | | NO | (Nitric Oxide or Nitrogen Monoxide) | | N2O | (Nitrous Oxide or Dinitrogen Monoxide) | | NO2 | (Nitrogen Dioxide) | | NO2- | (Nitrite Ion) | | NO2Cl | (NO2Cl) | | NO2F | (NO2F) | | NO3- | (Nitrate Ion) | | NOBr | (Nitrosyl Bromide) | | NOCl | (Nitrosyl Chloride) | | NOF | (Nitrosyl Fluoride) | | O2 | (Oxygen Gas, also called Elemental Oxygen) | | O22- | (Perioxide Ion) | | O3 | (Ozone) | | O3 | O3 Resonance Structures | | OCl2 | (OCl2) | | OCN- | (Cyanate Ion) | | OCS | (OCS) | | OF2 | (Oxygen Difluoride) | | OH- | (Hydroxide Ion) | | PBr3 | Phosphorus Tribromide | | PBr5 | Phosphorus Pentabromide | | PCl3 | Phosphorus Trichloride | | PCl4- | PCl4- | | PCl5 | Phosphorus Pentachloride | | PF3 | Phosphorus Trifluoride | | PF5 | Phosphorus Pentafluoride | | PF6- | Hexafluorophosphate Ion | | PH3 | Phosphorus Trihydride | | POCl3 | Phosphoryl Chloride or Phosphorus Oxychloride | | PO33- | (Phosphite Ion) | | PO43- | (Phosphate Ion) | | SBr2 | (Sulfur Dibromide) | | SCl2 | (Sulfur Dichloride) | | SCl4 | (Sulfur Tetrachloride) | | SCN- | (Thiocyanate) | | SeF4 | (Selenium Tetrafluoride) | | SeF6 | (Selenium Hexafluoride) | | SeO2 | (Selenium Dioxide) | | SF2 | (Sulfur Difluoride) | | SF4 | (Sulfur Tetrafluoride) | | SF6 | (Sulfur Hexafluoride) | | S2Cl2 | (Diulfur Dichloride) | | SiCl4 | (Silicon Tetrachloride) | | SiF4 | (Silicon Tetrafluoride) | | SiF62- | (Silicon Hexafluoride Ion) | | SiH4 | (Silicon Tetrahydride) | | SiO2 | (Silicon Dioxide) | | SnCl2 | (Tin (II) Chloride) | | SOCl2 | (SOCl2) | | SO2 | (Sulfur Dioxide) | | SO3 | (Sulfur Dioxide) | | SO32- | (Sulfite Ion) | | SO42- | (Sulfate Ion) | | Water | (H2O) | | XeCl4 | Xenon Tetrachloride | | XeF2 | XeF2 | | XeF4 | Xenon Tetrafluoride | | XeF6 | Xenon Hexafluoride | | XeH4 | XeO4 | | XeO3 | XeO3 | | XeO2F2 | XeO2F2 |
| Steps for Writing Lewis Structures - Find the total valence electrons for the molecule. Explain How Examples: H2S, NCl3, OH-
- Put the least electronegative atom in the center.
Note: H always goes outside.
Examples: NOCl, CF2Cl2, HCN
- Put two electrons between atoms to form a chemical bond. Examples: CH4, NH3, I2
- Complete octets on outside atoms.
Note: H only needs two valence electrons.
- If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.
Examples: O2, N2, C2H4
Advanced Steps - If you have extra electrons after the above steps add them to the central atom. Note: elements in the Period Three (usually S, P, or Xe) can have more than eight valence electrons.
Examples: ClF3, SF4,XeH4
- Check the Formal Charges to make sure you have the best Lewis Structure. Explain How
Examples: SO42-, N2O, XeO3
Notable Exceptions to the Octet Rule - H only needs 2 valence electrons.
- Be and B don’t need 8 valence electrons.
- S and P sometimes have more than 8 val. Electrons.
- Elements in Period Three, Four, etc (on the periodic table) can hold more than 8 valence electrons.
|
Lewis Dot Structure For Nh3


